The curtain has fallen on two fantastic tradeshows, AHR Expo and World of Concrete, and we’re still buzzing with excitement! We want to extend a heartfelt thank you to all the amazing customers, partners, and teammates who made these events unforgettable. Continue reading “Reflections: AHR Expo, World of Concrete, and What’s Next!”
Precision on Demand: Dwyer’s Online Product Configurator and Fast Track Program
 In the dynamic landscape of industrial instrumentation, every application has its unique demands. We want to ensure that our products meet your application needs. To do that, Dwyer Instruments provides not only a user-friendly online product configurator, but also a Fast Track Program for a fully customized solution.
In the dynamic landscape of industrial instrumentation, every application has its unique demands. We want to ensure that our products meet your application needs. To do that, Dwyer Instruments provides not only a user-friendly online product configurator, but also a Fast Track Program for a fully customized solution.
Continue reading “Precision on Demand: Dwyer’s Online Product Configurator and Fast Track Program”
Mitigating Methane in Landfills
 In the ongoing narrative of environmental conservation, few adversaries are as concerning as methane gas emissions from landfills. This potent greenhouse gas poses a substantial threat to both our climate and community health, demanding immediate attention and sustainable solutions.
In the ongoing narrative of environmental conservation, few adversaries are as concerning as methane gas emissions from landfills. This potent greenhouse gas poses a substantial threat to both our climate and community health, demanding immediate attention and sustainable solutions.
Landfills contain several different gases and volatile organic compounds (VOCs) caused by the breakdown of waste, with methane and carbon dioxide making up over 90% of them (health.ny.gov). Once waste breaks down and forms landfill gas and particulates, it can potentially spread to nearby buildings or structures through openings like cracks, windows, or ventilation systems and settle in areas of poor ventilation like basements or crawl spaces (health.ny.gov). Continue reading “Mitigating Methane in Landfills”
Flow Measurement with Orifice Plates
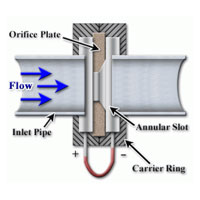
Dwyer Instruments offers many types of flow measuring products including orifice plates, which are used as a flow sensing element with a differential pressure monitor.

Orifice plates are a primary flow element, detecting the flow of a fluid passing through the plate by sensing the pressure drop across the plate. When a fluid flows through a restriction in a pipe, it creates a pressure difference between upstream and downstream of the restriction. This pressure difference is proportional to flow rate according to Bernoulli’s principal, similar to a Pitot tube. Orifice plates are commonly used as they are simple to use, low cost, work with gases or liquids, and require low maintenance. Adversely, they do have large pressure losses with about 50% of the pressure drop not recoverable. Continue reading “Flow Measurement with Orifice Plates”
Monitoring Solutions for Semiconductor Subfabs
A semiconductor transistor is a part with specific electronic properties that allow it to serve as a component in microchips and modern electronics like phones, laptops, and more. As these components are small and require precise manufacturing methods, there are facilities dedicated to their manufacture.
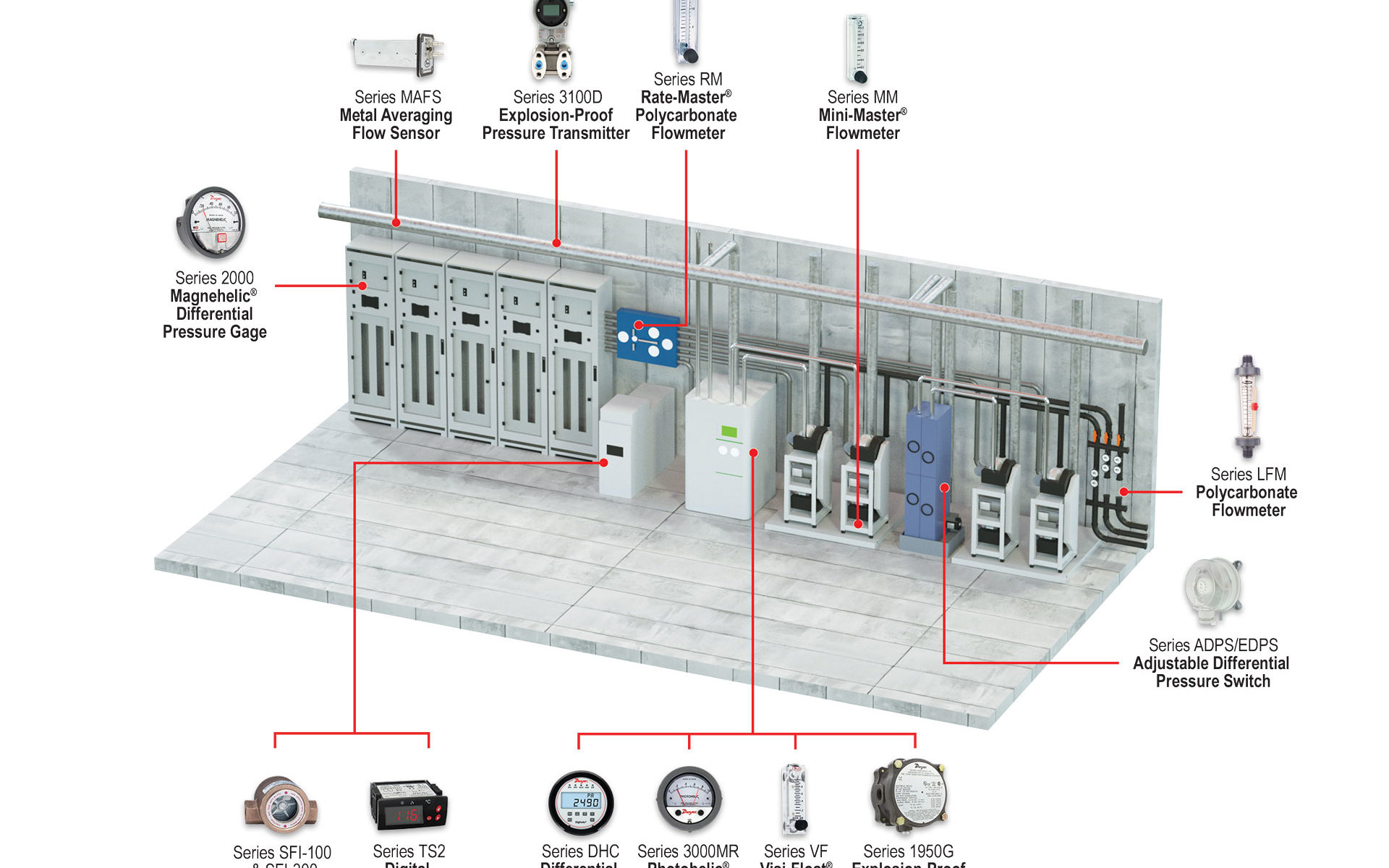
These facilities consist of several levels including air handlers and scrubbers for exhaust, HEPA room, fab cleanroom, and subfab areas. The control of pressure, flow, and temperature within the facility is essential.
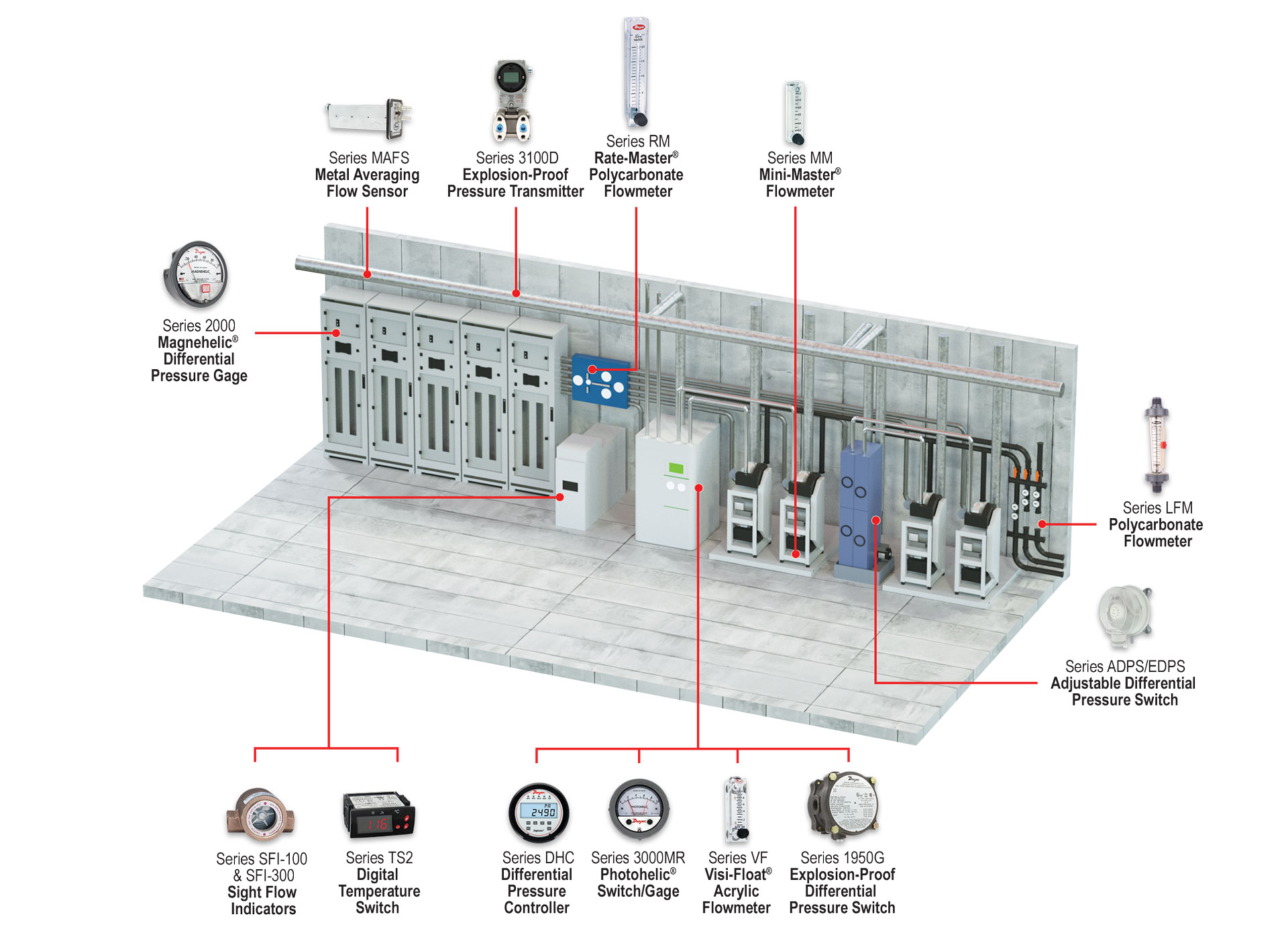
Semiconductor subfabs are located directly below the cleanroom. Here you will find an array of ancillary equipment, such as vacuum pumps, abatement systems, chillers, gas cabinets, and other equipment to keep process tools functioning efficiently. The equipment within the subfab is interconnected with the tools found within the fab cleanroom itself; these separate but connected areas work together to make sure the facility runs smoothly. Continue reading “Monitoring Solutions for Semiconductor Subfabs”