 The Dwyer team recently released the Series MSX Pro Magnesense® Differential Pressure Transmitter, which meets stringent industry standards with innovative global product design. This series is ideal for monitoring building control applications, and also utilizes enhanced accuracy and stability for use in high performance, critical environments. Continue reading “Product Highlight: Series MSX Pro Magnesense® Differential Pressure Transmitter”
The Dwyer team recently released the Series MSX Pro Magnesense® Differential Pressure Transmitter, which meets stringent industry standards with innovative global product design. This series is ideal for monitoring building control applications, and also utilizes enhanced accuracy and stability for use in high performance, critical environments. Continue reading “Product Highlight: Series MSX Pro Magnesense® Differential Pressure Transmitter”
Importance of Sensor Stability in Healthcare Isolation Rooms

The purpose of a healthcare isolation room is to prevent patients with contagious illnesses from spreading to others or to keep immunocompromised patients safe from exposure to airborne pathogens. As such, there exist two types of isolation rooms, either positively or negatively pressurized.
Positive pressure isolation rooms are designed to keep pathogens and outside air from entering the room, i.e. air inside the room is forced outward and is typically used for immunocompromised patients. Like clean rooms, it is important to maintain proper positive pressure within the protective isolation room to keep the patients safe. Continue reading “Importance of Sensor Stability in Healthcare Isolation Rooms”
What is Stability and Why is it Important?
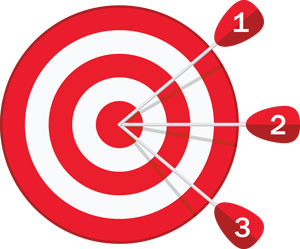 Stability is defined as a change (or lack of change) in accuracy over a period of time.
Stability is defined as a change (or lack of change) in accuracy over a period of time.
Drift is commonly used as a specification to illustrate the stability, or change in accuracy over a period of time, commonly shown as X%/year where X = a number; i.e. 0.25%/year. In this scenario, a device with a ±1% accuracy, would be expected to have an accuracy of ±1.25% (1%+0.25%) after a period of one year. Depending on the design, brand, and range of the sensing instrument, the stability can vary widely. Continue reading “What is Stability and Why is it Important?”
Happy Holidays | Year in Retrospect

As the year comes to a close and we celebrate this time with our families and friends, all of us at Dwyer want to give a big thank you to the customers and teammates that we’ve depended on throughout the year. We certainly couldn’t have done it without you.
This year has been unlike any other, to say the least. The COVID-19 pandemic shook the world as hospitals and emergency personnel struggled to keep up with demand for ventilators and isolation rooms. Worldwide manufacturing slowed, as non-essential businesses shut down and companies shifted to CDC guidelines and recommendations. Continue reading “Happy Holidays | Year in Retrospect”
USP Guidelines for Compounding Facilities
 The United States Pharmacopeia (USP) is a non-profit organization that develops standards for human and animal drugs as well as food ingredients and dietary supplements.
The United States Pharmacopeia (USP) is a non-profit organization that develops standards for human and animal drugs as well as food ingredients and dietary supplements.
For pharmaceutical compounding facilities, USP has guidelines in four general chapters:
- USP <795> Pharmaceutical Compounding – Nonsterile Preparations
- USP <797> Pharmaceutical Compounding – Sterile Preparations
- USP <800> Hazardous Drugs – Handling in Healthcare Settings
- USP <825> Radiopharmaceuticals – Preparation, Compounding, Dispensing, and Repackaging
These four chapters provide guidelines for safety considerations, personnel qualification and training, facilities and engineering controls, microbiology and surface monitoring, cleaning and disinfection, and much more. Continue reading “USP Guidelines for Compounding Facilities”



